
Henri Cramail
Full professor

Sustainable development is a major challenge for the chemical industry of the XXIst century. It must reconcile science, technology, economic viability and citizen acceptability. In this context, the use of biomass as a source of polymer precursors (building blocks) is of growing interest for both the academic and industrial communities. It is first one of the converging solutions to minimize the environmental impact of polymer-based materials and technologies. Biomass can also be the starting point of innovative pathways for designing new tailor-made building blocks and original macromolecular architectures. Indeed, the diversity and the specificities (e.g. hetero-functions) of the building blocks derived from biomass are opportunities to develop new polymers with properties that are unprecedented in petroleum-based materials.
Among the manifold bioresources used for the purpose of polymer chemistry, our team developed a very strong expertise in the use of derivatives stemming from: (i) vegetable oil (triglycerides) and (ii) lignin (phenolic substrates such as vanillin). Following the concepts of green chemistry, we are using these synthons to develop new routes for the synthesis of: (i) polyurethanes, (ii) polycarbonates and (iii) epoxy thermosets, three of the cardinal polymer families.
One of our most representative contributions to this research topic is the use of triglyceride derivatives for the synthesis of polyurethane precursors, notably aliphatic polyamines and poly(cyclic carbonate)s. The polyaddition of the latter through the aminolysis of the cyclic carbonates is offering an isocyanate-free route to polyurethane [1]. In this perspective, we developed new routes for the synthesis of polyamines and poly(cyclic carbonate)s starting from aliphatic backbones derived from fatty acids [2]. In the case of poly(cyclic carbonate)s, the carbonate functions were derived from glycerol which is, with fatty acids, the other constituent of triglycerides [3,4]. With these new biobased precursors in hands, we addressed several limitations of the synthesis of polyurethanes based on the polyaddition of amine and cyclic carbonate. Notably, we demonstrated that the carbonate-amine reaction can be accelerated, and match the kinetics of the conventional isocyanate-alcohol chemistry, by activating the cyclic carbonate with hetero-functions in β-position of the cycle (e.g. ether or ester) [5].
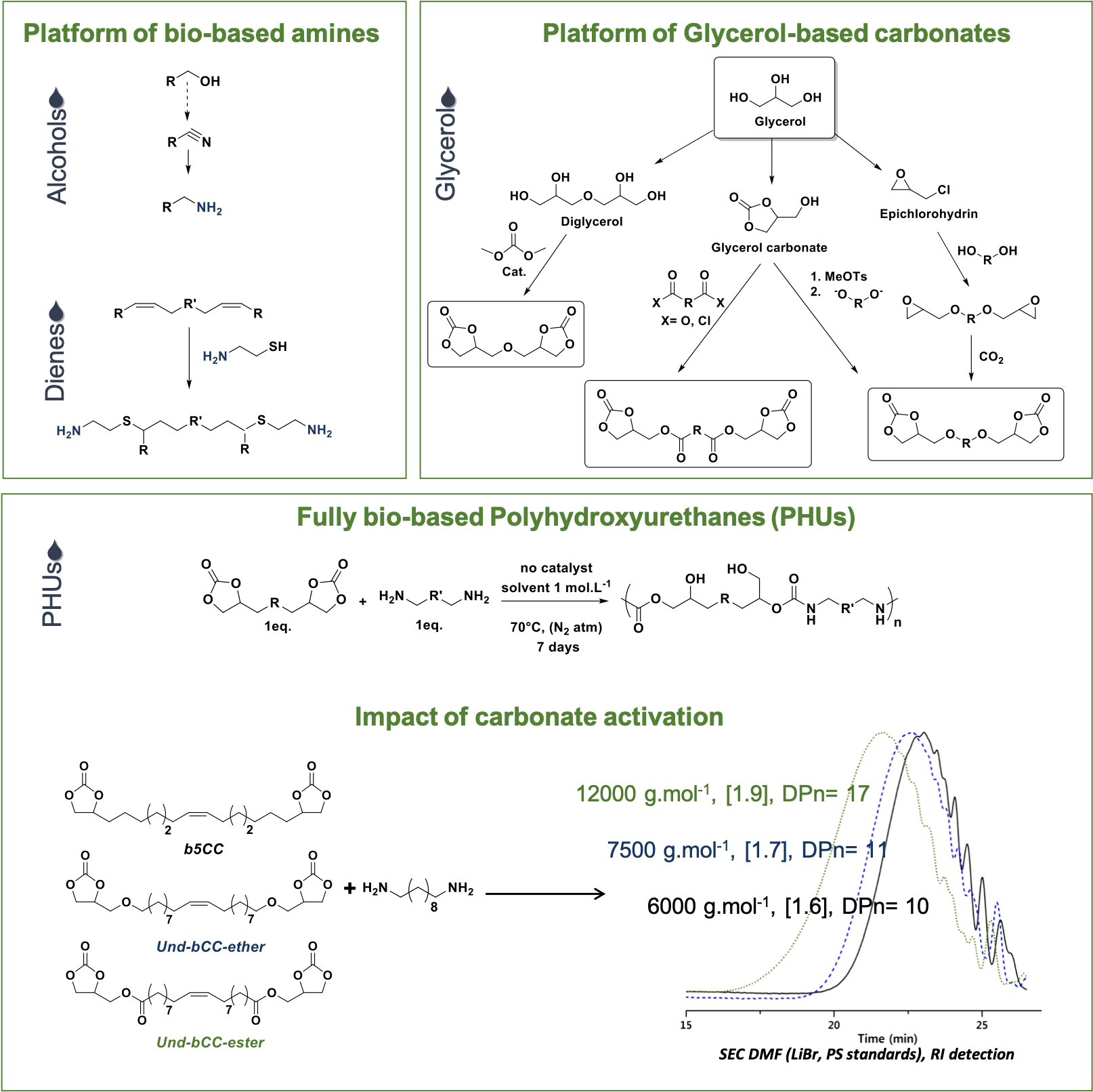
[1] L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau, H. Cramail, Chem. Rev., 115(22), 12407-12439, (2015)
[2] G. Hibert, O. Lamarzelle, L. Maisonneuve, E. Grau, H. Cramail, Eur. Polym. J., 82, 114-121 (2016)
[3] L. Maisonneuve, A. S. More, S. Foltran, C. Alfos, F. Robert, Y. Landais, T. Tassaing, E. Grau, H. Cramail, RSC Adv., 4 (49), 25795–25803, (2014)
[4] O. Lamarzelle, G. Hibert, S. Lecommandoux, E. Grau, H. Cramail, Polym. Chem., 8(22), 3438-3447, (2017)
[5] O. Lamarzelle, P-L. Durand, A-L. Wirotius, G. Chollet, E. Grau, H. Cramail, Polym. Chem., 7(7), 1439 (2016)